Hydrogen explosion in test chambers - a game with fire

On November 3, 2022, a violent explosion of a lithium-ion battery at Innovapark in Kaufbeuren caused considerable building damage, destroying a complete outer wall of a hall. Fortunately, no people were harmed. But how did this happen?
The fire behavior of large batteries for electromobility through to entire vehicles is examined in greater detail in test halls where a short circuit is provoked. The fact that this can lead to major explosions in addition to a fire is a new aspect, however, which does not leave the requirements for the test hall operators entirely cold. Even a battery fire places high demands on safety. The energy released in a fire is in the single-digit gigajoule range. The fire power is several megawatts. If the entire vehicle is burned, both the energy and the power can reach double digits. Additional explosion loads are therefore extremely unwelcome.
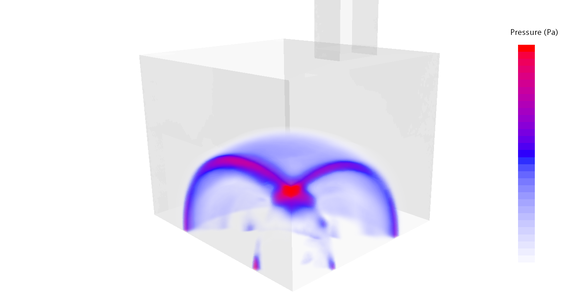
Explosions can also occur when hydrogen collects in a bubble, which can then explode abruptly. The resulting pressure wave can destroy entire buildings, as can be seen.
In a CFD simulation, we can model the behavior of hydrogen or any other explosive and determine the compressive forces acting on the walls. How the building behaves is shown by a subsequent FEM calculation in which the pressure loads are taken from the CFD simulation.

The typical bearable loads of common concrete walls are in the range of 10-20 kN/m². This corresponds to a pressure of only 0.1 - 0.2 bar. In the event of an explosion of a gas under ambient pressure, the volume increases approximately 8-fold, which corresponds to a maximum achievable theoretical pressure of 8 bar. Too much for any normal building; after all, buildings are not designed as pressure vessels. The smaller the test chamber and the closer the explosion source is to the wall, the higher the pressure peaks of the explosion wave when it hits the wall. The explosion can be accurately described in a suitable CFD flow program, based on the chemical reactions taking place. Not only the duration of the explosion and the pressure curve on the walls, taking into account the reflections of the pressure waves, can be determined in this way, but also the resulting temperatures.
The simulation even shows when unburned gas escapes to the environment due to a lack of oxygen, it finds oxygen again here in the ambient air and ignites again. I once had a Mini Cooper Works that could be heard from far away when you stepped off the gas and the unburned gasoline vapors ignited with fresh ambient air at the exhaust. So what does not speak for the engine builder, but that's just by the way :)
The legitimate question can be asked as to how a larger hydrogen bubble can form at all in the flaming inferno of a burning battery. This can happen when there is not enough oxygen available for combustion.
In principle, explosive gas mixtures have a so-called lower and upper explosion limit. Too little or too much fuel prevents an explosion.
If one examines the explosion of a gas bubble in an oxygen-containing space, the chemical reaction takes place at the surface of the bubble, comparable to a burning ball. If one of the two reaction partners runs out, either the fuel or the oxygen, the chemical reaction comes to a standstill.
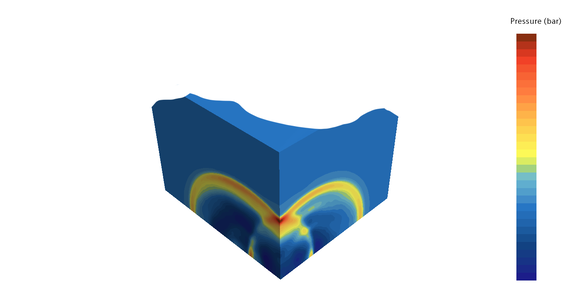
Since unburned hydrogen is very light, it collects in bubbles, especially in niches in the ceiling, where it cannot escape upwards unhindered. Test chambers for testing batteries should therefore tend to have a funnel shape that is open at the top and should be well exhausted.
Even the old Hindenburg would probably not have exploded if the hydrogen escaping from a leak had not been able to collect in gaps in the hull, but had simply escaped upwards.
We would be happy to help you with the design and protection of test halls. We could also tell you how your building will collapse if the worst comes to the worst because you have underestimated the issue. Regarding the test hall in Kaufbeuren, we can say on the basis of the pictures in the press that the building walls are certainly not suitable for bearing larger explosion loads. Reinforced concrete with somewhat thicker walls would be a more suitable building material. But the operators themselves certainly know that by now.
By the way, did you know that the electrical fire load of a lithium-ion battery is about a factor of 10 greater than the stored electrical power? And again, it's worth taking a look at the energy densities in Wiki.
We will be happy if we can also help you to build test chambers only once.
Yours Stefan Merkle
PS: You want to specifically blow up old ammunition in a container? We'll show you what happens and whether and how it works. But you probably don't want to do that anyway, do you? Just in case, you can see our contact details below.
PPS: You have built a new test center for batteries and are not quite sure if you can sleep peacefully? We look forward to hearing from you.
PPPS: Are you planning a new test center for batteries? You should definitely talk to us.
